DIVA Celebrates 30th Anniversary with Three Milestone Product Launches and New Website Debut

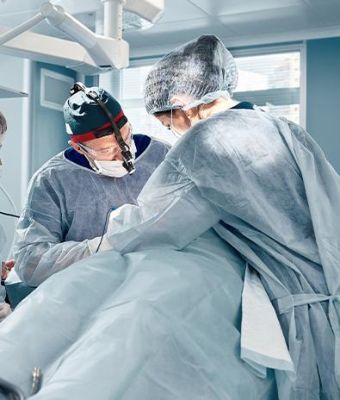
DIVA celebrates its 30th anniversary with the debut of its new corporate website and mass production of industry-first optical bonding displays for surgical use. Pictured: Vice Chairman & CEO Mr. Gene Chen (left) and General Manager Ms. Yvonne Liu (right) at the exhibition booth.
[Taipei, Taiwan — July 2025] DIVA, a professional medical display manufacturer with 30 years of experience, announces three major product developments that reinforce its commitment to high-performance imaging solutions. The industry’s first 55” and 65” optical bonding (OB) LCDs designed specifically for surgical environments have entered mass production, recognized by top-tier international customers. The new QDII product line, positioned as an Off-The-Shelf (OTS) platform, will begin shipments in July to meet the growing demand for flexible and cost-efficient display solutions in entry-level medical and industrial applications. OLED displays have been successfully integrated into ultrasound imaging systems, overcoming long-standing challenges with burn-in and panel lifespan. Coinciding with its 30th anniversary, DIVA has launched a new corporate website to showcase its customized display capabilities. The new site provides a more intuitive interface for customers to explore product portfolios, accelerating the consultation and conversion process.
Large-size Optical Bonding: Advancing Surgical Display Performance
Large-size displays have long presented challenges for medical integration, particularly in surgical settings where glare reduction, dark-level uniformity, and bonding yield are critical. Partnering with the Qisda Group, DIVA optimized structural design and bonding processes, pioneering the use of optical bonding (OB) in 55” and 65” LCDs for surgical environments. The displays also feature AGAR (Anti-Glare, Anti-Reflective) glass, reducing ambient light interference to support high-brightness, high-contrast performance in catheter labs and endoscopic operating rooms.
QDII Platform: Standardized, Scalable, and Efficient
To address the needs of system integrators and distributors working in entry-level medical and industrial display segments, DIVA introduced the QDII series as an Off-The-Shelf (OTS) platform. Leveraging Qisda’s panel inventory and production resources, QDII supports streamlined supply planning through shared tooling and integrated manufacturing capacity. While positioned for cost efficiency, the series maintains professional-grade mechanical and quality control standards, reflecting DIVA’s commitment to reliable, scalable solutions.

OLED in Ultrasound: Stability Through Proprietary Control
OLED displays offer exceptional contrast and response times, but adoption in real-time medical imaging has been limited by concerns over image retention and panel longevity. DIVA has overcome these challenges by applying proprietary image control technologies—including Off RS, Orbit, and JB algorithms—to achieve greater stability and image consistency.
This development enables broader integration of OLED into ultrasound imaging systems, with future potential for expansion into other medical domains.
Quote from Leadership
“Medical displays are like the second pair of eyes for healthcare professionals during life-critical moments. They must deliver both precision and durability,” said Ms. Yvonne Liu, General Manager of DIVA Labs. “Our OB-based large-size displays for surgical rooms represent a first-of-its-kind breakthrough, and this recognition by leading global customers affirms DIVA’s design and manufacturing excellence.”